Colorado family's fight for livesaving medication for infant with Barth syndrome leads to FDA approval
After months of fighting, a Colorado family is breathing a sigh of relief after the FDA announced initial approval of an experimental medication helping to keep their son alive.
"Like last night, I had the idea, 'Oh, I could watch football.' (laughs) But I didn't -- I was too tired, " Madison Dryden said.
After nine months of racing the clock, that kind of peace is new for the Drydens.
"I put a countdown on my watch face, and it was counting down to Nov. 4. So, each day, I'd look and say, 'Okay, one less,'" Andy Dryden said.
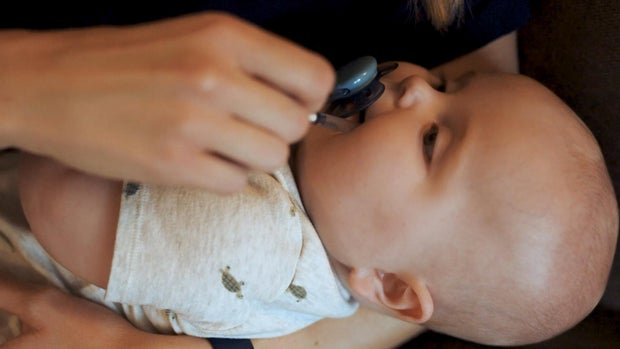
They had been counting the days until the FDA decided the fate of a drug their son desperately needed to survive. Gilbert was born with Barth syndrome, a rare and often fatal mitochondrial disease.
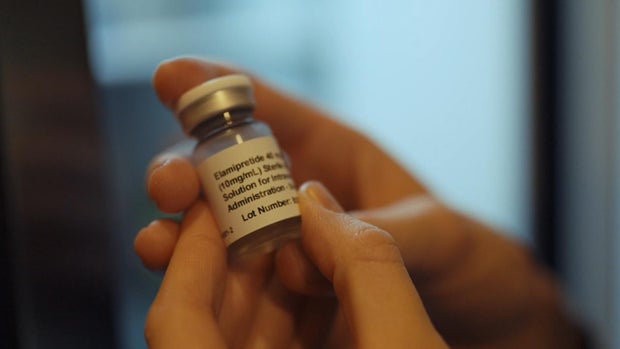
Just days old and in heart failure, he was granted emergency access to the drug elamipretide. Without FDA approval, their supply would eventually end.
"Before it was like, 'Okay, what are we going to do for his next round of medication?'" Andy said.
The remaining vials marked the days of stability the family had left, and the Drydens began their fight, calling lawmakers, posting online, and doing everything they could to save the drug.
"Even with all those concrete actions, it was still a feeling of, 'Okay, we have this many days. What's going to happen if they say no again? What's our plan?'" Madison said, looking back on that time.
One week before the expected decision, Madison received a message.
"It kind of felt like, 'Is this real? Is this too good to be true?'" she said.
Elamipretide was approved.
"It took a couple of days to sink in, now we are feeling really excited and hopeful about everything," Madison added.
The approval means Gilbert and other patients in critical need can continue their treatment.
"Emotionally, I think the other thing has been gratitude -- just really thankful for everyone that showed up for us," she said.
With time now on their side, the Drydens are beginning to imagine life without the constant pressure.
"I'm anticipating that I'll start to have normal thoughts again, and I'm looking forward to that," Andy said.
Madison believes their case proves one thing clearly: the FDA can move quickly when time matters.
"This shows that it is possible for the FDA to move quickly. It shows that it is possible for the FDA to make decisions that are helpful for the treatment of kids who have very limited options and very limited life expectancy," she said.
The director of research at the FDA, in a statement following the approval, said: "The FDA remains committed to facilitating the development of effective and safe therapies for rare diseases and will continue to work diligently to help ensure patients with rare diseases have access to innovative treatments."
The Drydens say while they do plan to try and get some rest, they are turning their attention to advocating for other families still waiting for treatment.